Hatcheries are the starting point of poultry production, and their role in disease prevention is critical. Among the most effective tools for protecting flocks and ensuring food safety is vaccination. When combined with strict biosecurity and hygiene measures, vaccination helps prevent outbreaks, improves flock performance, and reduces public health risks associated with contaminated poultry products.
This article explores strategies and practices for vaccination and disease control in hatcheries, focusing on vaccine handling, preparation, administration methods, and essential protocols to ensure success.
Why Vaccination Is Essential in Hatcheries?
Vaccination is not just a routine step—it is a strategic intervention that provides immunity against major poultry diseases such as Marek’s disease, Newcastle disease (ND), Infectious Bronchitis (IB), Gumboro disease and Infectious Laryngotracheitis (ILT). Effective vaccination programs:
- Protect vulnerable chicks during their most susceptible stage.
- Reduce economic losses caused by mortality, poor growth, and treatment costs.
- Enhance food safety by minimizing zoonotic pathogens in poultry products.
However, vaccination alone cannot guarantee success. It must be integrated into a comprehensive disease control program that includes biosecurity, cleaning, and disinfection throughout the production cycle.

Core Principles of Effective Hatchery Vaccination
To achieve optimal results, hatcheries should follow these principles:
- Correct Vaccine Selection
Choose vaccines based on local disease prevalence and production systems. Consultation with regional poultry health specialists is essential. - Proper Storage and Handling
Vaccine potency depends on strict adherence to supplier guidelines for storage and handling. - Accurate Administration
Use calibrated equipment and trained personnel to ensure correct dosage and minimize stress on chicks. - Documentation and Traceability
Record all vaccine-related data for compliance and troubleshooting.
1-Vaccines storage : maintaining potency
Vaccines and treatments should be stored in appropriate conditions, in suitable quantities considering the forecasted needs of the hatchery and supply time. Vaccine efficiency might be reduced if the conditions of storage, according to suppliers’ recommendations, are not applied.
Two Main Storage Methods :
- Liquid Nitrogen (-196 °C)
Used for frozen vaccines. Nitrogen levels should be checked regularly, and operators must wear proper protective equipment when handling liquid nitrogen. - Refrigeration
For vaccines stored at controlled temperatures. Monitor temperature regularly, and discard any vaccine if storage conditions are uncertain.

2-Vaccines preparation : a critical step
Proper preparation ensures vaccine integrity and effectiveness. Key guidelines include:
- Dedicated Preparation Area
- Separate from chick handling rooms, clean, disinfected, and restricted to authorized personnel.
- Strict Hygiene
- Handwashing and disinfection before handling vaccines are mandatory.
- Inspection of Vials and Diluent
- Check diluent color—discard if it is yellowish or cloudy.
- Ensure vials are stored head-down. If vaccine is present in the vial head-top, discard it because freezing process was interrupted.
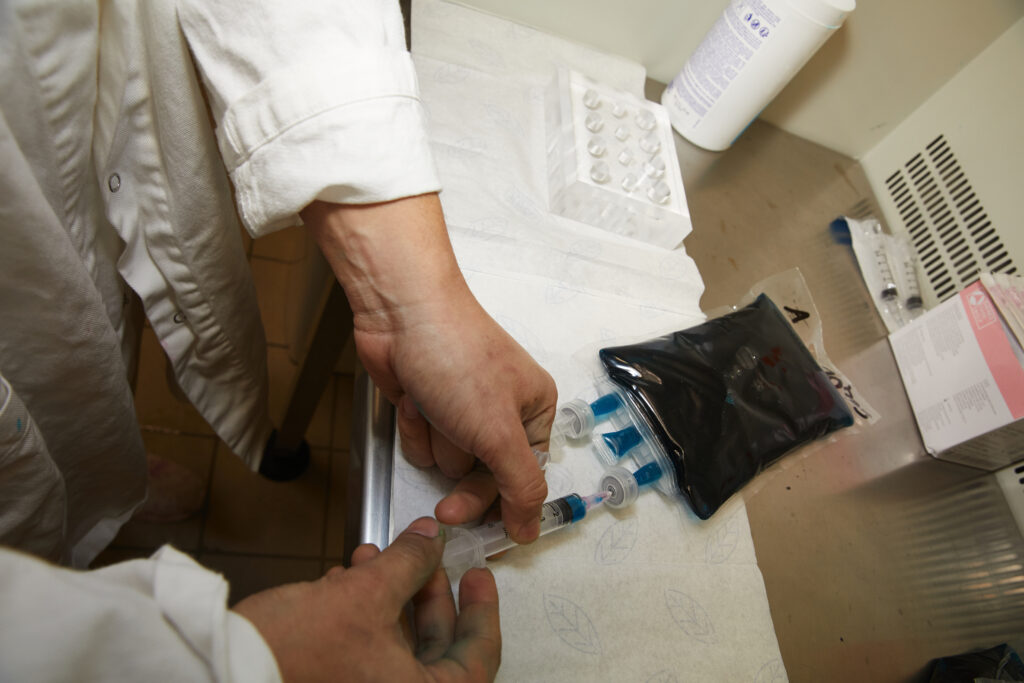
3-Preparation of Frozen Vaccines
Follow these steps to maintain vaccine integrity:
Step Diagram: Vaccine Preparation Process
Thaw the vial
Place the glass vial in a water bath at 27 °C for no more than 90 seconds.
⬇️
Dry the ampoule thoroughly
Ensure the ampoule is completely dry before proceeding.
⬇️
Slowly draw up the vaccine
Use a sterile syringe with a needle of at least 18G to slowly aspirate the vaccine from the ampoule.
⬇️
Inject the vaccine into the diluent bag
Inject the vaccine slowly into the diluent bag
⬇️
Rinse and swirl
Rinse the tip of the glass ampoule with the diluent, then gently swirl the diluent bag to ensure thorough mixing
4-Preparation of Refrigerated Vaccines
Like frozen vaccines, refrigerated vaccines require careful handling to maintain their potency. Follow these best practices:
- Temperature Control: Keep vaccines within the recommended range (usually 2–8 °C). Avoid temperature fluctuations during handling.
- Dedicated Preparation Area: Prepare vaccines in a clean, disinfected space separate from chick handling zones.
- Avoid Direct Sunlight: Never expose vaccines to direct sunlight or high ambient temperatures during preparation.
- Mixing: Reconstitute vaccines using suitable sterile diluent or water bottle. Mix gently to avoid damaging live organisms.
- Timing: Once reconstituted, use the vaccine within the time specified by the supplier (typically 30–90 minutes).
- Verify dilution according to chick box count: Incorrect dilution can lead to underdosing or overdosing, compromising vaccine effectiveness and flock health.
5-Vaccine Registration and Traceability
Recording vaccine data is essential for compliance and problem-solving. Below is the information to be included in the documents:
- Vaccine type, batch number, and expiry date.
- Nitrogen level and refrigerator temperature.
- Time of preparation and completion.Number of vials used and chicks vaccinated.
- Names of operators responsible for preparation.
Methods of Vaccine Administration
Hatcheries use several administration techniques; each suited to specific vaccines and supplier recommendations.
1. In-Ovo Vaccination
Performed when eggs are transferred from the setter to the hatcher (18–19 days). Requires precise injection into the egg and strict hygiene. When combined with proper preparation and biosecurity, in-ovo vaccination is highly effective.
2. Day-Old Chick Injection
Administered subcutaneously (back of the neck) or intramuscularly (leg muscle) immediately after hatching. Key requirements:
- Trained personnel and calibrated equipment.
- Needle hygiene: Replace every 1,000 chicks.
- Use reconstituted vaccine within 60 minutes.
3. Spray Vaccination
Used for respiratory vaccines (IBV, NDV) and live coccidiosis vaccines. Critical factors:
- Control water dosage and droplet size.
- Ensure uniform coverage.
- Follow supplier recommendations for equipment settings.

Biosecurity and Hygiene: The Foundation of Success
Vaccination cannot compensate for poor biosecurity. Hatcheries must implement:
- Access control to vaccine preparation areas.
- Regular cleaning and disinfection of equipment and workspaces.
- Personal protective equipment (PPE) for all operators.
Standard Operating Procedures (SOPs)
Developing an SOP manual ensures consistency and compliance. It should cover the follozing:
- Vaccine reception and storage.
- Preparation steps for each vaccine type.
- Administration techniques.
- Hygiene and safety protocols.
- Documentation requirements.
Regional Adaptation and Expert Consultation
No single vaccination program fits all regions. Disease prevalence varies by geography, climate, and production systems. Therefore:
- Consult local specialists for tailored programs.
- Monitor disease trends and adjust schedules.
- Train staff continuously on best practices.
CONCLUSION
Vaccination and disease control in hatcheries are essential for sustainable poultry production. Success depends on precision, consistency, and strict adherence to protocols—from vaccine storage and preparation to administration and documentation. By combining effective vaccination strategies with strong biosecurity and expert guidance, hatcheries can protect flocks, optimize performance, and contribute to global food security.
